Do you know that you can use light to determine the concentration of a solution? Beer-Lambert law, a law formulated in 1913 by Robert Luther, and Andreas Nikolopulos from laws discovered by Pierre Bouguer (but attributed to Johann Heinrich Lambert) and August Beer, let us find the concentration of a solution from the absorbance value measured by a spectrophotometer.
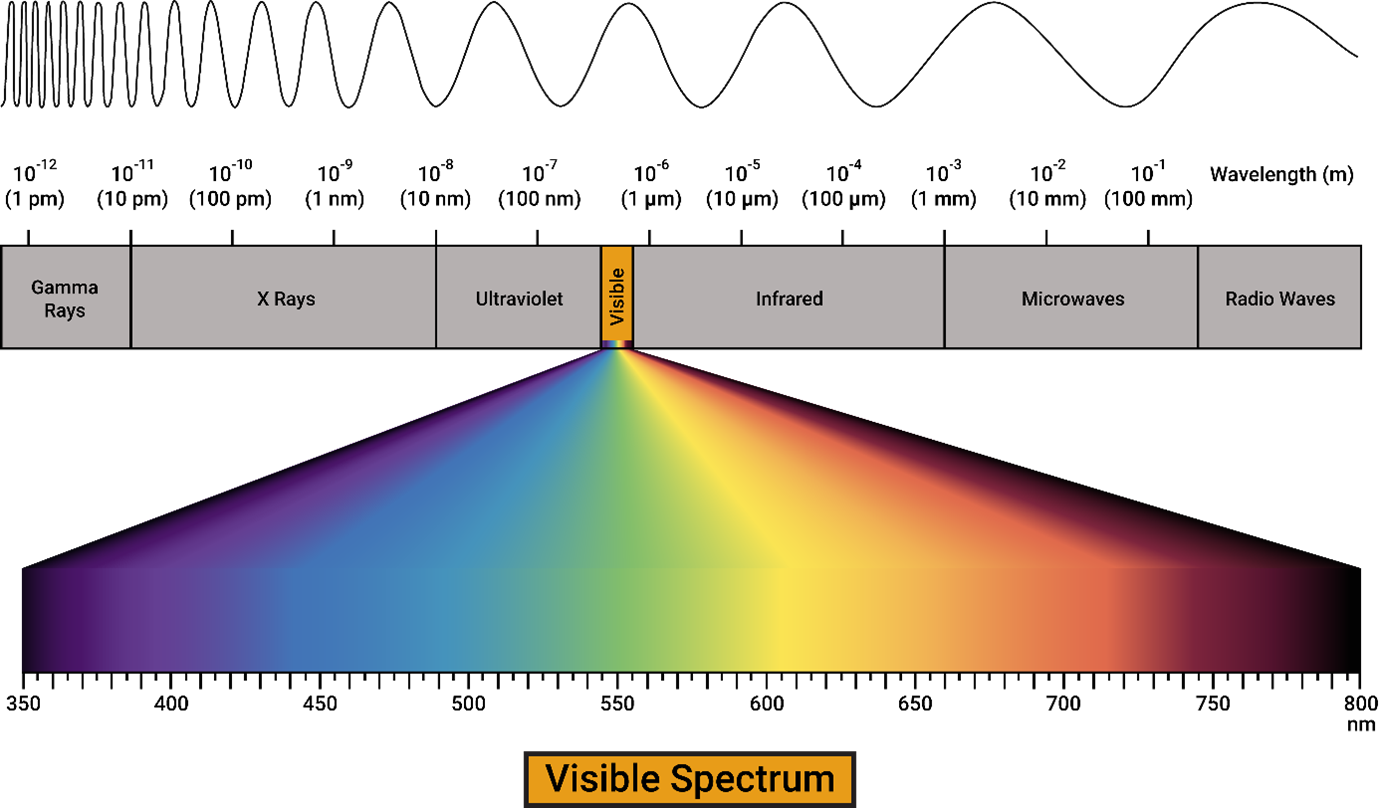
Figure 1. Electromagnetic spectrum
Beer-Lambert Law lets us determine the concentration of a substance with the data acquired from a UV-Vis spectrophotometer. The visible light spectrum is also called the UV-Vis spectrum. The edge closer to the Infrared region has the lowest energy whereas the edge closer to the Ultraviolet region has the highest energy within the UV-Visible region of the electromagnetic spectrum. We see the color of a substance because light interacts with the substance. When a certain wavelength of light is absorbed by a substance, the rest is emitted. The emitted region accounts for the color of the substance. The color of a solid object depends on the intensity of light it absorbs and reflects within the UV-vis region of the electromagnetic spectrum. In the case of colored solutions, the intensity of incident light, I, and the intensity of the transmitted light, I0at a certain wavelength are important. Transmittance is defined by the ratio, I/I0. I and I0are measured by a special device named UV-Vis Spectrophotometer.
The first commercially available spectrophotometer was invented by National Technical Laboratories owned by the chemist, inventor, and investor Dr. Arnold Orville Beckman. Its sale started in 1941 with the name “DU Spectrophotometer”. Dr. Arnold O. Beckman was also behind the invention of the pH meter and the commercialization of the IR (Infrared) Spectrophotometer and O2analyzer. [i] The commercial availability of these scientific instruments let them accessible to modern science laboratories and facilitated numerous scientific discoveries. In other words, Dr. Arnold O. Beckman revolutionized instrumental chemistry for quantitative analysis.
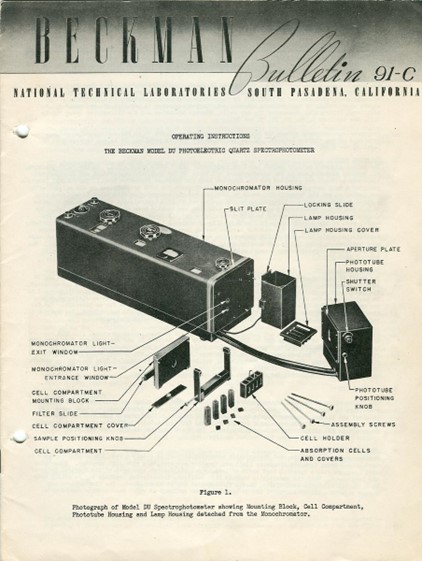
Figure 2. DU Model Spectrophotometer by National Technical Laboratories [ii]
Nowadays, much more advanced versions of DU Spectrophotometer are available in various sizes, shapes, and prices but its availability to education laboratories is still questionable. Hence, VRLab Academy combined empirical data and theoretical formulation to bring about a virtual laboratory experience called “UV-Vis Spectrophotometer and Beer-Lambert Law” compatible with guided inquiries suggested by AP (Advanced Placement) for Chemistry. Book a demo meeting to explore this essential instrument and more with VRLab Academy, and enhance your science education with us.
[i] https://doi.org/10.1021/ac041608j
[ii] Beckman Manual,1954