Before the Rutherford the atomic model was known as Thomson’s model which the structure of the atom was a sphere of positive charge housing smaller negatively charged electrons within it, like plums within a pudding. In 1911, Rutherford and coworkers Hans Geiger and Ernest Marsden initiated a series of groundbreaking experiments that would completely change the accepted model of the atom. This experiment which proved the existence of a nucleus in the atom and changes the understandig of atomic structure.
Rutherford insisted on a model with a high charge concentrated in the middle of the atom, also, containing the mass of the atom- nucleus (Planetary Orbit Model). He tested the validity of the Thomson atomic model by conducting the following experiment:
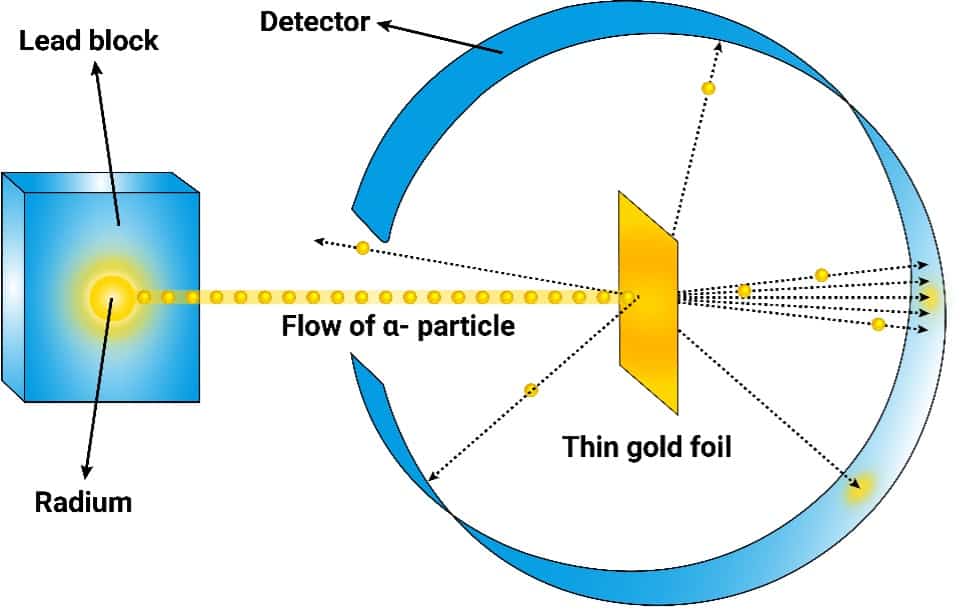
1.He prepared the experimental setup in Figure 1 consisting of an α-particle source (positively charged He2+), a thin gold foil (0.6 μm), and a ZnS detector.
2.He bombarded the gold foil. If the Thomson model was right the α-particles would be expected to have enough vacant space to penetrate the foil and hit the detector unobstructed.
3.However, he observed that some α-particles were changing angles.
4.Then, concluded that there were points on the gold foil where positive mass was concentrated (atomic nucleus). Negatively charged electrons were assumed to move around this nucleus.
In short for Rutherford experiment the following are true:
- Positive charge is concentrated in the nucleus of the atom.
- The mass of the atom is almost equal to the mass of the nucleus.
- Negatively charged electrons move around the nucleus.
- Electrons are very small in size compared to the nucleus, they are distributed around the nucleus and occupy most of the volume of the atom.
- The atoms are neutrally charged because the positively charged nucleus and the negatively charged electrons compensate each other.

Rutherford's model proved to be a significant step towards a full understanding of the atom. However, it did not fully clarify the nature of electrons and how they occupied the huge space surrounding the nucleus. A full understanding of the electron became possible only a few years later. According to the experiment results proved to be the key to understanding the chemical properties of elements.
Through VRLab Academy, students can have hands-on experience with a virtual lab in VRLab Academy that shows the atomic models . They can learn much as Ernest Rutherford did, using scientific principles in an online simulation. They can do the same experiment a number of times, or try out different versions. This way, you will get new experiences and acquire new knowledge. This is an experiment that changes the world for students!
Take a look at all experiments at VRLab Academy andenhance your teaching power with us.
References
https://www.radioactivity.eu.com/site/pages/Rutherford_Experiment.htm
https://www.nobelprize.org/prizes/chemistry/1908/rutherford/biographical/